Draw the best Lewis structure for CH3CHCHCHCHCHCOOH a neutral molecule. Connect the exterior and core central atom of the NCl3 molecule with three single N-Cl bonds.
Solved Draw The Lewis Structure Of The Following Molecule Chegg Com
Formal charges are optional.
. Draw the Lewis structure of COF2. Chemistry questions and answers. Draw the Lewis structure of NCl3.
FREE Expert Solution Valance electrons. See the answer See the answer done loading. Include lone pairs NCl3.
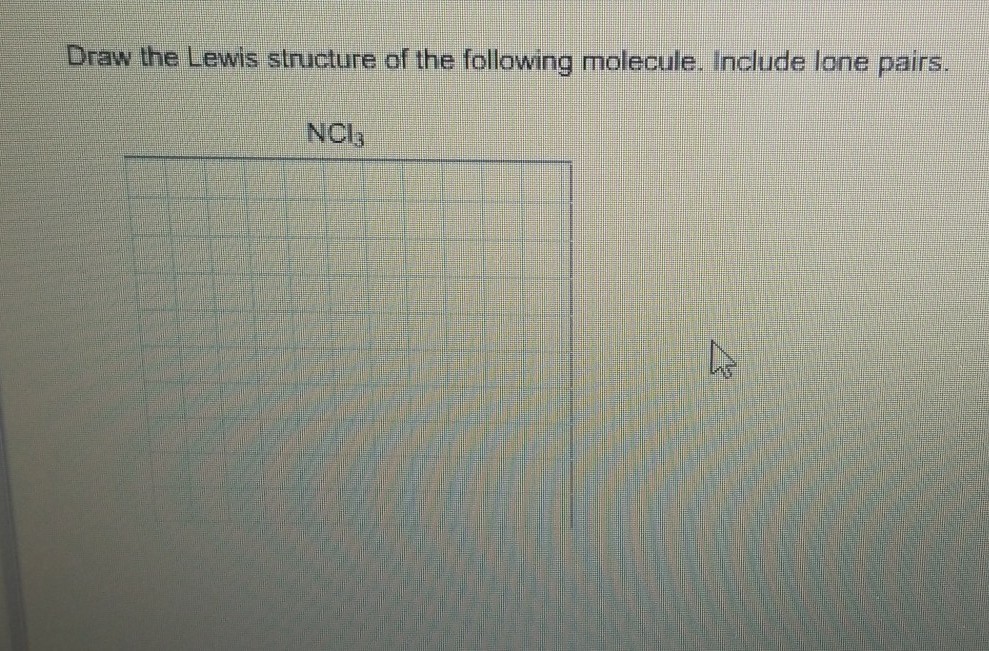
Draw the Lewis structure of CO23CO32. Nitrogen in the NCl3 Lewis structure with all three chlorine atoms arranged in a trigonal pyramidal geometry. Add valence electrons around the chlorine atom as given in the figure.
Select Draw Rings More III Si H. Draw the Lewis structure of the following molecule. Draw a Lewis structure for each of the following molecules.
Draw the Lewis structure of SiH4. Draw the Lewis structure of the following molecule. Draw the Lewis structure by placing atoms on the grid and connecting them with bonds.
Include all lone pairs of electrons. Draw the Lewis structure of HCNHCN. CH2O C2Cl4 CH3NH2 CFCl3 C.
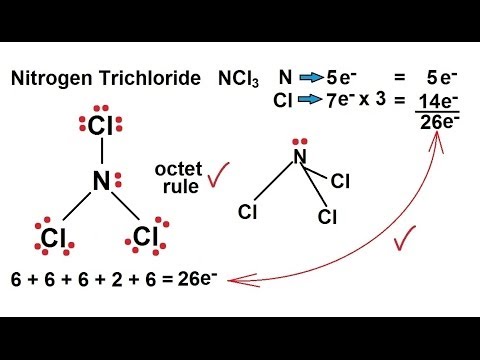
Nitrogen trichloride NCl3 contains one nitrogen and three chlorine atoms. Include all lone pairs of electrons and nonbonding electrons. Therefore the total number of valence electrons in butyne 1 5 3 7 26.
Lewis structure of NCl 3 can be drawn by using valence electrons of nitrogen and chlorine atoms. Include lone pairs as needed. Draw the Lewis structures of the given molecules.
100 4 ratings Answer. Include all lone pairs of electrons. Learn this topic by watching Lewis Dot Structures.
This problem has been solved. NF3 HBr SBr2 CCl4 Write a Lewis structure for each molecule. OF CO2 Select Draw Rings More Erase Select Draw Rings More Erase 0 F С.
There are one lone pair on nitrogen atom and three lone pairs on each chlorine atom. Include lone pairs on all atoms where appropriate. Include lone pairs NCl3.
In the image the red color dots are the valence electrons of. Include lone pairs on all atoms where appropriate. View the full answer.
Formal charges are optional. Select Draw Rings More F o с. The given molecule is As we know that nitrogen has 5 valence electrons and chlorine has 7 valence electrons.
Draw the Lewis structure of CO23CO32. HF Н0 Select Draw Rings More Erase Select Draw Rings More 2 3 HNO Select Draw Rings More Erase 2 o Draw the Lewis structure for each molecule. Draw the Lewis structures of the given molecules.
Show transcribed image text. Each chlorine atom has three lone pairs of electrons while nitrogen has one lone pair of electrons. Lewis structure of COF2.
Draw the Lewis structure of NCl3. 0 5 Complete these structures by adding electrons in the form of dots as needed. According to Lewis-dot structure there are 6 number of bonding electrons and 20 number of non-bonding electrons.
See the answer See the answer See the answer done loading. The molecule geometry of the molecule is trigonal pyramidal while. Silicon have 4 valence electrons and hydrogen.
Draw the Lewis structure of the following molecule. Include all lone pairs. Lewis dot Structure for NCl3 generated from step-1 and step-2.
View the full answer. Nitrogen trichloride NCl 3 lewis structure contains three N-Cl bonds. OF CO2 Select Draw Rings More Erase Select Draw Rings More Erase.
Draw the Lewis Structure for 2-butynal. Draw the Lewis structure of HCNHCN. Show transcribed image text Expert Answer.
Chemistry Chemical Bonding 14 Of 35 Lewis Structures Nitrogen Trichloride Ncl3 Youtube

Ncl3 Nitrogen Trichloride Lewis Structure

Ncl3 Lewis Structure Molecular Geometry Bond Angle Polarity Electrons

Ncl3 Lewis Structure How To Draw The Dot Structure For Ncl3 Youtube

Draw The Lewis Structure Of The Following Molecule Include Lone Pairs Ncl3 Brainly Com

Ncl3 Lewis Structure How To Draw The Dot Structure For Ncl3 Youtube

Solved Draw The Lewis Structure Of Nci Include Lone Pairs Chegg Com

0 comments
Post a Comment